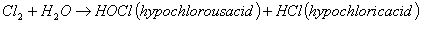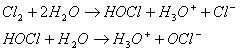|
Index page |
|
|
TASK 2CHLORINATION
History of chlorine as a disinfectant:
Chlorine was discovered in 1774 by the chemist Carl Wilhelm Scheele, who mistakenly thought it contained oxygen. Then in 1810 Humphry Davy gave the name chlorine to this element (from the Greek language Chloros, meaning “pale green”). Today chlorine is used widely in the manufacture of many items:
One of the first known uses of chlorine for water disinfection was by John Snow in 1850, when he attempted to disinfect the Broad Street Pump water supply in That is why today chlorine is one of the most widely used disinfectants. It is used to kill bacteria and other microbes from water to avoid diseases caused by microorganisms. How does chlorine work to disinfect?
When chlorine is added to water, this reaction takes place:
Hypochlorous acid is the active, killing form of chlorine. Depending on the pH value, hypochlorous acid partly expires to hypochlorite ions (OCl-):
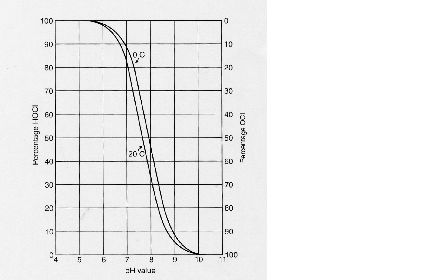
Theses compounds (HCl and OCl-) are called free chlorine molecules. A molecule is free when it has not bonded with or combined with another compound. So, these molecules are available for disinfecting. They kill microorganisms y slashing through the cell walls and destroying the inner enzymes, structures and processes. The microorganisms will either die or suffer from reproductive failure. But hypochlorous acid is more reactive and is a stronger disinfectant than hypochlorite ions. In fact, it is split into hydrochloric acid (HCl) and atom air oxygen (O). The oxygen atom is a powerful disinfectant. However, when chlorine is added to water for disinfection purposes, it usually starts reacting with dissolved organic and inorganic compounds in the water. These compounds are called “active chlorine compounds”. For example, chlorine can react with ammonia (NH3) to chloramines. After that, chlorine can no longer be used for disinfection because it has formed other products. The amount of chlorine that is used during this process is referred to as the “chlorine enquiry” of the water. Chlorination and the pH value: As the pH of the water increases, the killing power of the chlorine decreases. Disinfection with chlorine takes place optimally when the pH is between 5,5 and 7,5. Indeed, at this value, hypochlorous acid (the most reactive) is mainly present in the water. With a pH value of 6 the level of hypochlorous acid is 80%, whereas the concentration of hypochlorite ions is 20%. When the pH value is 7,5, concentrations of hypochlorous acid and hypochlorite ions are equally high.
Different types of chlorine: § Gas (Cl2): most commonly used form of chlorine. This toxic, yellow-green gas is stored as a liquid under pressure. § Sodium hypochlorite solution (NaOCl): It is a clear, light yellow, highly alkaline, and corrosive with a strong chlorine odor. It contains 5 to 15% of chlorine. § Calcium hypochlorite (Ca(OCl)2): It is a white, highly corrosive and dry solid that contains 70% of chlorine. § Bromide chloride (BrCl): Bromide residuals are less lethal to aquatic life than that of chlorine compounds. What dose of chlorine applies? The dose has to be high enough for a significant amount of chlorine to remain in the water for disinfection. Chlorine enquiry is determined by the amount of organic matter in the water, the pH of the water, the contact time and exposure.
Advantages of chlorination: § It is a well-established technology. § Presently, chlorine is more cost-effective than either UV or ozone disinfection. § The chlorine residual can prolong disinfection even after initial treatment and can be measured to evaluate the effectiveness. § Chlorine disinfection is reliable and effective against a wide spectrum of pathogenic organisms. § Chlorine is effective in oxidizing certain organic and inorganic compounds. § Chlorination has flexible dosing control. § Chlorine can eliminate certain noxious odors while disinfecting. Disadvantages:
§ All forms of chlorine are highly corrosive and toxic. § Chlorine oxidizes certain types of organic matter, creating more hazardous compounds (for example: trihalomethanes, THMs). § Some parasitic species have shown resistance to low doses of chlorine (including oocysts of Cryptosporidium parvum, cysts of Endamoeba histolytica and Giardia lamblia, and eggs of parasitic worms). According to the World Health Organization (WHO), disinfection by chlorine is still the best guarantee of microbiologically safe water.
You can send comments : aub@niras.dk
Started by NIRAS supervisor Sergio Fox on 27th March 2006. © COPYRIGHT 2001 ALL RIGHTS RESERVED Aurelie.dk |