|
Index page |
|
|
|
TASK 2
IMPROVEMENTS OF CHLORINATION
We can replace chlorine by bromine or ozone, but those pose also problems (WHO, 2000). Only the system copper-money, used in two swimming pools in ü Simple modifications:
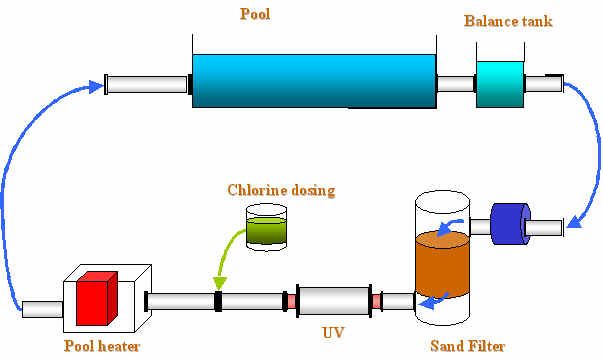
However some improvements can be made with chlorination. To reduce chloramine formation, it is necessary to reduce the organic matter contribution, that is to say to act on the hygiene of the bathers (shower ideally soaped, passage to the toilets before going to water for the children, effective port of the bathing cap...). It is also important to control chlorine concentrations in water, air and water temperature. An increase of the water temperature facilitates the volatilisation of chlorinated product into the air. Besides to avoid the accumulation of the chlorinated products in the air, the ventilation system needs to be efficient (avoiding the recycling of the air). Systems of degasification apart from the halls of swimming pool can also be installed. ü Chlorination with UV disinfection:
An other technique can be chlorination associated with UV disinfection. By the use of an UV disinfection system, the concentration of chloramines is significantly reduced. During several tests with UV disinfection on municipal swimming pools, the overall chlorine consumption was reduced with an average of 50% without an increase of bacterial counts in the water. In that way, the concentration of chloramines was both reduced by the breakdown of the formed chloramines by Ultra Violet light and by the lower chlorine dosage. An additional advantage of this system is the reduced aging of the fabrics in and around the swimming-pool.
Moreover, a French research by the INRS has shown that the use of this system can contribute to an increase in the risk of exposure to products such as the haloformes (chloroform, dichlorométhane...) suspected to be carcinogenic. It does not bring a satisfactory response to the problems due to nitrogen trichloride (NCl3). ü Stripping:
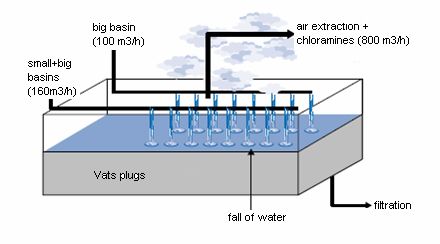
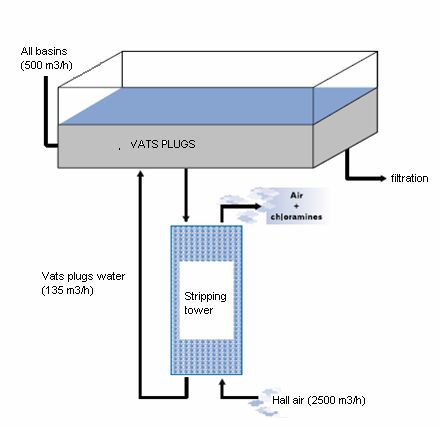
This method can be used for the extraction of chloramines. As we have seen, chloramines are irritating pollutants and they are (especially trichloramine) volatiles. It is thus easily to extract them by stripping. It consists in putting in contact a liquid and a gas and to reach a thermodynamic balance, that is to say to reach a stationary state between the liquid phase and the gas phase. This concept of thermodynamic balance makes it possible to describe how several components are distributed between a liquid phase and a vapour phase, and to know if the contact time is sufficient to reach the stationary state. Certain owners and originators of swimming pools chose to use this technique to treat a part of their water of basins, by ventilation on the level of the vats plugs which collect the overflows of the basins. These systems of ventilation of water are varied as you can see on these following drawings:
ü Example: The
In this indoor swimming pool, there were swimmers who consistently complained of skin irritation and eye burn. Besides, the indoor pool facility’s total air handling system showed marked deterioration due to the presence of high chloramine levels in the air. There were also signs of corrosion around the pool area. So, solutions must have been found. An aggressive superchlorination program was tried but it was a waste of time and money. In fact, the chloramine level decreased for 4 days or less and then it increased again. An ozone system with a non-chlorine shock was tested but it was unsuccessful. Some consultants have said that a superchlorination of the pool was required every night, but it was of course not feasible. Better ventilation and dilution with outside air was carried out: the air handling system was operated at 100%, air circulation devices were blown across the top of the pool, and a superchlorination took place 2 times per month. However, combined chlorine levels continued to run high and the air quality at the facility remained poor. Consequently, a new process control system designed to provide precise control for pool water chemistry and air quality in indoor aquatic facilities was tested. It was a Strantol Environmental Control System (ECS), manufactured by US Filter’s Stranco Products in Bradley. The ECS controls the rate of oxidation and also the types of oxidation reactions that take place in pool water. The system is designed to feed the optimum concentrations of oxidizer in order to prevent the formation of volatile chloramine. This is accomplished utilizing high resolution redox (HRR) technology, which directly and continuously measures the rate of oxidative disinfection that can be affected by organic load. In fact, the formation of volatile chloramine is prevented by the optimized and controlled rate of oxidation of all organic compounds entering the pool. The ECS also calculates the Ryznar Index, which indicates the corrosive tendencies of the water and computes the required chemical addition needed to properly balance pool water. Benefits of this process: · It consistently maintained combined chlorine levels in the pool water at < 4,0 ppm (combined chlorine levels in the dehumidification system’s condensate were undetectable) · It reduced maintenance costs associated with extending equipment life, cleaning pool surfaces and resurfacing of the pool. · The improvement in water and air quality had completely eliminated the need for superchlorination, thus eliminating costly and inconvenient shutdowns · Complaints of skin rashes, eye burn and bleached suits had been eliminated. · There were cost savings in term of chemical consumption.
You can send comments: aub@niras.dk Started by NIRAS supervisor Sergio Fox on 27th March 2006. © COPYRIGHT 2001 ALL RIGHTS RESERVED Aurelie.dk |