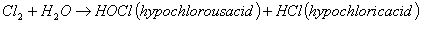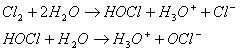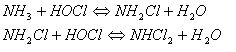|
Index page |
Task6 (index) |
TASK 6SPA PROJECT IN COPENHAGEN Chlorination and health problems (reminding)
One of the first known uses of chlorine for water disinfection was by John Snow in 1850 in When chlorine is added to water, this reaction takes place:
These molecules (HOCl and OCl-) are called free chlorine molecules. So, they are available for disinfecting. They kill microorganisms by slashing through the cell walls and destroying the inner enzymes, structures and processes. The microorganisms will either die or suffer from reproductive failure. Hypochlorous acid is more reactive and is a stronger disinfectant than hypochlorite ions. In fact, it is split into hydrochloric acid (HCl) and atom air oxygen (O). The oxygen atom is a powerful disinfectant. However, when chlorine is added to water for disinfection purposes, it usually starts reacting with dissolved organic and inorganic compounds in the water. These compounds are called “active chlorine compounds”. For example, chlorine can react with ammonia (NH3) to chloramines. After that, chlorine can no longer be used for disinfection because it has formed other products. Consequently, the dose of chlorine to apply must be high enough for a significant amount of chlorine to remain in the water (after reaction with dissolved compounds). The chlorine enquiry is a function of the amount of organic matter in the water, the pH, the contact time and the exposure. Some researchers say that chlorine has some very serious health consequences when used as a sanitizer in swimming pools. Effects of chlorine on human health depend on the concentration of chlorine present in air, and on the duration and frequency of exposure. They also depend on the health of an individual and the environmental conditions during the exposure. Chlorine enters the body breathed in with contaminated air or when consumed with contaminated food or water. When small amounts of chlorine are breathed in during short time periods, it can affect the respiratory system (coughing, chest pains, and fluid accumulation in the lungs). Chlorine can also cause skin and eye irritations. In fact, it is not chlorine that is dangerous for the health but its by-products: chloramines and trihalomethanes (THMs). The chloramines are responsible for the smell and the irritant properties of the swimming-pool air. Thus, when swimmers complain of too much chlorine in the pool area, the real problem is too little free chlorine in the water (too much chlorine has been converted to chloramines). These chloramines, monochloramine (NH2Cl), dichloramine (NHCl2) and trichloramine (NCl3) are generated from the reaction of HOCl with ammonia and amino-compounds that originate from sweat and urine of the swimmers.
They are (especially the trichloramine) quite volatile and they partition easily from the water into the air. The main factors that determine the production and the air levels of these by-products are: the degree of water chlorination, the contamination of the water by nitrogen-containing compounds (which depends on the number of bathers, their behaviour and hygiene), water temperature and air recirculation. Some studies have identified trichloramine as the probable cause of respiratory problems and occupational asthma in swimmers and pool attendants, at indoor swimming pools. The trihalomethanes are also by-products of chlorination which can be harmless for the human health. They come from the substitution of the hydrogen atoms in methane (CH4) with the halogen atoms of chlorine or bromine. The methane is a natural product of our bodies and the products of this substitution called “THMs” are for example: ü CHCl3 Chloroform or trichloromethane ü CHBr3 Bromoform ü CHCl2Br Bromodichloromethane ü CHClBr2 Dibromochloromethane Like the chloramines, the rate of THM production is a function of the number of swimmers, the total organic carbon content of the water, the pH and the water temperature. Besides, chloroform can be measured in the blood or urine of regular swimmers.
As a consequence, it is necessary to control the formation of the chlorine by-products by having swimmers wash before entering, by treating the water, by maintaining adequate water exchange rates, and by constant dilution of the air above indoor swimming pools via good ventilation practises. Another alternative could be to use another disinfection technique. You can send comments : aub@niras.dk
Started by NIRAS supervisor Sergio Fox on 27th March 2006. © COPYRIGHT 2001 ALL RIGHTS RESERVED Aurelie.dk |